Recent advances in the study of 3D protein structures have fundamentally transformed our understanding of biological mechanisms and human diseases. Structural biology techniques, such as X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy (cryo-EM), now allow for the determination of protein structures with increasing precision, from atomic-level resolution to complex macromolecular assemblies.
These methods are essential for deciphering how structural changes in proteins contribute to diseases, and for the design of targeted therapeutic interventions.
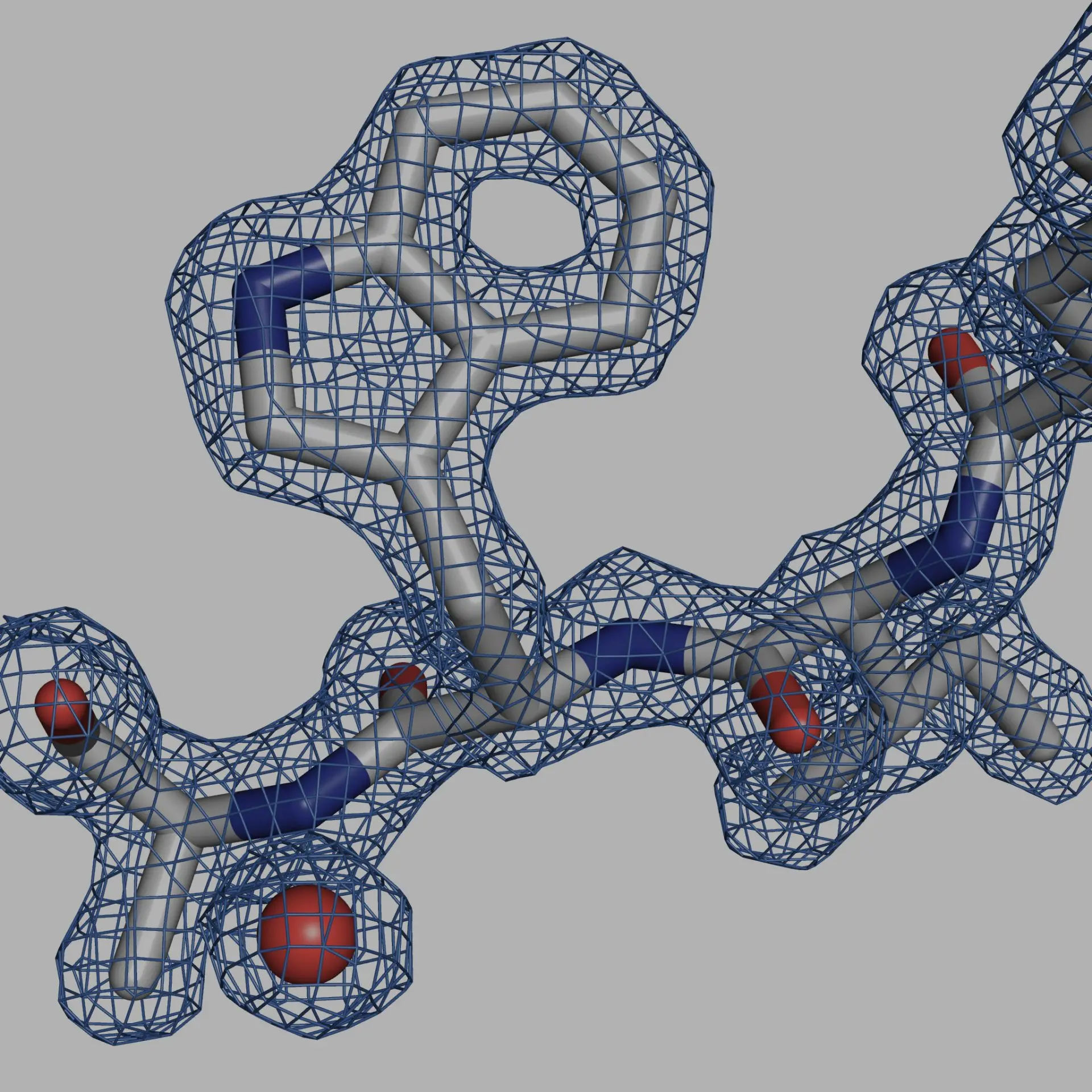
1. Cryo-EM for High-Resolution Structural Analysis
Cryo-EM has revolutionized structural biology by enabling the high-resolution study of large protein complexes and membrane proteins without the need for crystallization. This technique has enabled the visualization of proteins in their native, functional state, making it possible to study dynamic and heterogeneous structures that were previously inaccessible. For example, cryo-EM was crucial in determining the structure of the SARS-CoV-2 spike protein, leading to rapid vaccine development.
2. X-ray Crystallography: A Gold Standard for Precision
X-ray crystallography remains a gold standard for obtaining highly detailed protein structures at atomic resolution. This technique allows the determination of protein structures with precision, essential for understanding molecular interactions and designing drugs. However, it requires the formation of high-quality protein crystals, which can be challenging for flexible or dynamic proteins. Despite these limitations, it remains a vital tool for many structural studies, particularly for well-ordered proteins like enzymes or signaling molecules.
2. X-ray Crystallography: A Gold Standard for Precision
X-ray crystallography remains a gold standard for obtaining highly detailed protein structures at atomic resolution. This technique allows the determination of protein structures with precision, essential for understanding molecular interactions and designing drugs. However, it requires the formation of high-quality protein crystals, which can be challenging for flexible or dynamic proteins. Despite these limitations, it remains a vital tool for many structural studies, particularly for well-ordered proteins like enzymes or signaling molecules.
3. NMR Spectroscopy for Studying Proteins in Solution
NMR spectroscopy is a powerful tool for studying proteins in solution, providing insights into their native conformation and interactions. Unlike X-ray crystallography and cryo-EM, NMR allows the study of proteins in their natural environment, which is critical for understanding proteins with flexible or transient structures. NMR data can also be combined with other structural techniques to provide a more complete understanding of protein function.
4. Molecular Modeling and Dynamic Simulations
Once 3D structures are obtained, molecular modeling and simulation tools allow the simulation of protein dynamics within its biological environment, providing essential information about flexibility, conformational changes, and interactions with other molecules. Software like GROMACS or AMBER enables molecular dynamics simulations at the atomic level, offering insights into protein dynamics in complex biological environments, such as cellular membranes or multi-enzyme complexes. These simulations also help predict the effects of mutations or post-translational modifications on protein function, facilitating the design of drugs that specifically target these sites.
5. Therapeutic Applications: From the Laboratory to the Clinic
One of the most promising applications of structural biology is the design of targeted drugs. A detailed understanding of 3D protein structures has enabled the discovery of new therapeutic targets and the design of small molecules, monoclonal antibodies, and peptides capable of specifically modulating the activity of proteins involved in diseases. For example, the structure of the PD-1 receptor involved in immune regulation and its interaction with the PD-L1 ligands led to the development of innovative cancer therapies, such as immune checkpoint inhibitors (e.g., pembrolizumab).
In neurodegenerative diseases like Alzheimer’s, the structure of amyloid-beta and associated complexes has provided insights into the formation of toxic aggregates, leading to the development of treatments aimed at inhibiting or removing these aggregates. Advances in understanding the structures of viral proteins, such as the SARS-CoV-2 spike protein, have also enabled the design of mRNA vaccines, marking a major breakthrough in the fight against global pandemics.
Current Challenges and Future Perspectives
Despite significant advances, challenges remain. Membrane proteins are still difficult to study due to their amphipathic nature and instability in aqueous environments. Cryo-EM and recent advances in protein stabilization techniques have made strides in addressing this, but further optimization is required. Additionally, modeling proteins with highly flexible or transient structures remains a challenge. The integration of artificial intelligence tools like AlphaFold offers exciting potential to predict protein structures from amino acid sequences, significantly accelerating structural biology research.